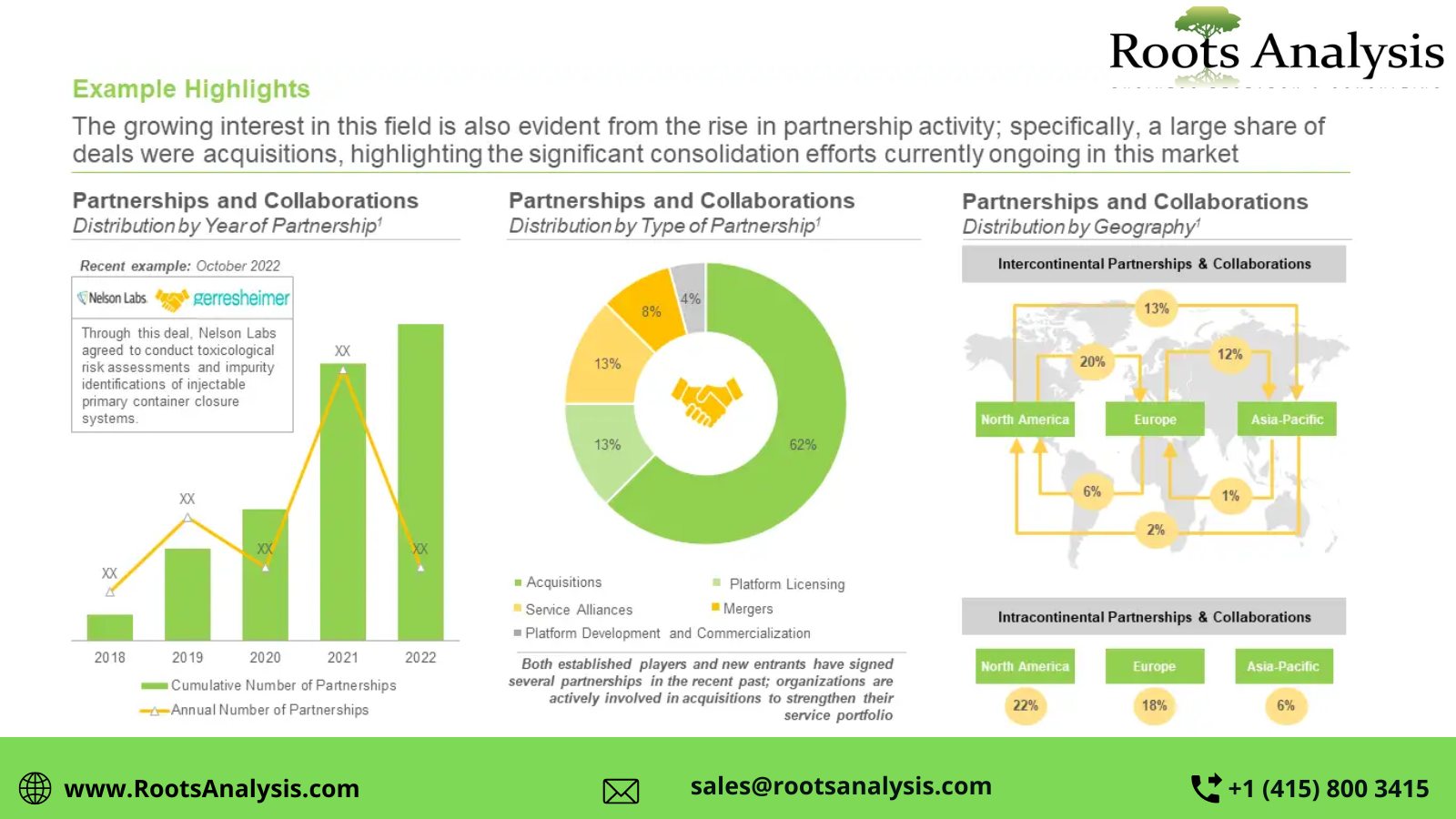
Genotoxicity Testing: Unlocking the Future Safety Assessment Opportunities
Genotoxicity testing refers to the evaluation of detrimental effects of chemical or physical agents on the genetic processes and related hereditary material of living cells.
On the other hand, mutagenicity is the process of inducing irreversible and transmissible alterations in the genetic material of organisms either spontaneously or through mutagenic activity.
Disruption of the integrity and function of DNA at the gene or chromosomal level can lead to heritable mutations, resulting in genetic disorders, birth abnormalities, or cancer. Potential targets for DNA damage include somatic cells (confined to the exposed generation), germinal cells (potentially inherited effects), and mitochondria (detrimental to the exposed individual and progeny via maternal inheritance).
Detrimental Effects of Genotoxins and Mutagens
Genotoxins are chemical substances or radiations which causes genotoxicity leading to DNA or chromosomal damage, further leading to mutations. In eukaryotic organisms, damage to genetic material of somatic cell can lead to malignancy (cancer) whereas genetic damage to germ cells can result in heritable mutations that induce birth defects.
Mechanism of Genotoxicity / Mutagenicity
The interaction of genotoxins / mutagens with the structure of DNA causes damage to the genetic material. These genotoxic / mutagenic substances interact with the DNA structure at a specific base sequence, inducing deletion, mis-segregation, or non-disjunction, resulting in damage and mutation.
Techniques Used for Genotoxicity / Mutagenicity Testing
Genotoxins can be classified based on their effects into the following types:
· Carcinogens: These have the ability to cause cancer. Examples – Asbestos, Benzene, Vinyl chloride and Carbon tetrachloride
· Mutagens: These can induce mutations in the genetic material of an organism. Examples – Chloroform, Ethylene oxide, and Lead
· Teratogens: These agents cause birth defects, abnormalities, or developmental problems in the offspring. Examples – Carbon monoxide, Lead and Xylene
GENE MUTATION
Ames Test: It is used to evaluate an agent’s mutagenic potential by reversing mutations in the tester mutant bacteria (E. coli, Salmonella typhi), as well as its ability to synthesize an essential amino acid required for growth
MLA / HPRT: It is used to evaluate an agent’s mutagenic potential by reversing mutations in the tester mutant bacteria (E. coli, Salmonella typhi), as well as its ability to synthesize an essential amino acid required for growth
Mutation Assay: Transgenic rodent somatic and germ cell gene mutation assay specifies an in vivo assay that perceives gene mutation causing agents. Transgenic mice or rats with several copies of chromosomally integrated plasmids or phage shuttle vectors are employed in this test.
DNA DAMAGES
Comet Assay: The comet assay, also known as single cell gel electrophoresis (SCGE), is an efficient technique for identifying DNA strand breaks in a cell. It is used in molecular epidemiology, genotoxicity testing, and basic studies on DNA damage and repair.
CHROMOSOMAL DAMAGES
Chromosomal Aberration Test: The comet assay, also known as single cell gel electrophoresis (SCGE), is an efficient technique for identifying DNA strand breaks in a cell. It is used in molecular epidemiology, genotoxicity testing, and basic studies on DNA damage and repair.
Micronucleus Test: The micronucleus identifies chemicals (liquid or solid) that lead to cytogenetic damage, resulting in the formation of micronuclei containing complete or lagging chromosomal segments.
Applications of Genotoxicity / Mutagenicity
Genotoxicity testing and mutagenicity testing can be employed in a wide range of industries. It detects the potential long-term effects of the compounds which are marketed without knowing their ability to affect human and environmental health.
Recent Development and Future Perspectives
Recent advancements in informatics and instrumentation technologies have facilitated the necessity to evaluate mutations and chromosomal damage caused by various chemicals. In addition, (Q)SAR models produced by industrial and non-industrial organizations are effectively employed to anticipate the mutagenicity and chromosomal damage of bacteria. Several new approaches are being developed for efficient mutagenic and genotoxic studies. These novel models can detect homologous recombination in the majority of the tissues of interest.
For additional details, please visit https://www.rootsanalysis.com/blog/genotoxicity-mutagenicity-testing/
You may also be interested in the following titles:
- Quantum Computing in Drug Discovery Services Market : - Industry Trends and Global Forecasts, 2023-2035
- Viral Clearance and Viral Testing Services Market : - Industry Trends and Global Forecasts, 2023-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com




